

Chemistry 103 Virtual Laboratories
Organic Chemistry
Our virtual laboratories provide students with immersive, hands-on science experiences without the limitations of physical lab spaces. Access cutting-edge experiments and simulations 24/7 from any device.
Each virtual lab is designed by experienced educators and scientists to align with curriculum standards while providing interactive learning opportunities that engage students in authentic scientific inquiry.
Available Virtual Labs
Explore our comprehensive collection of STEM laboratory simulations
Organic Chemistry Laboratories
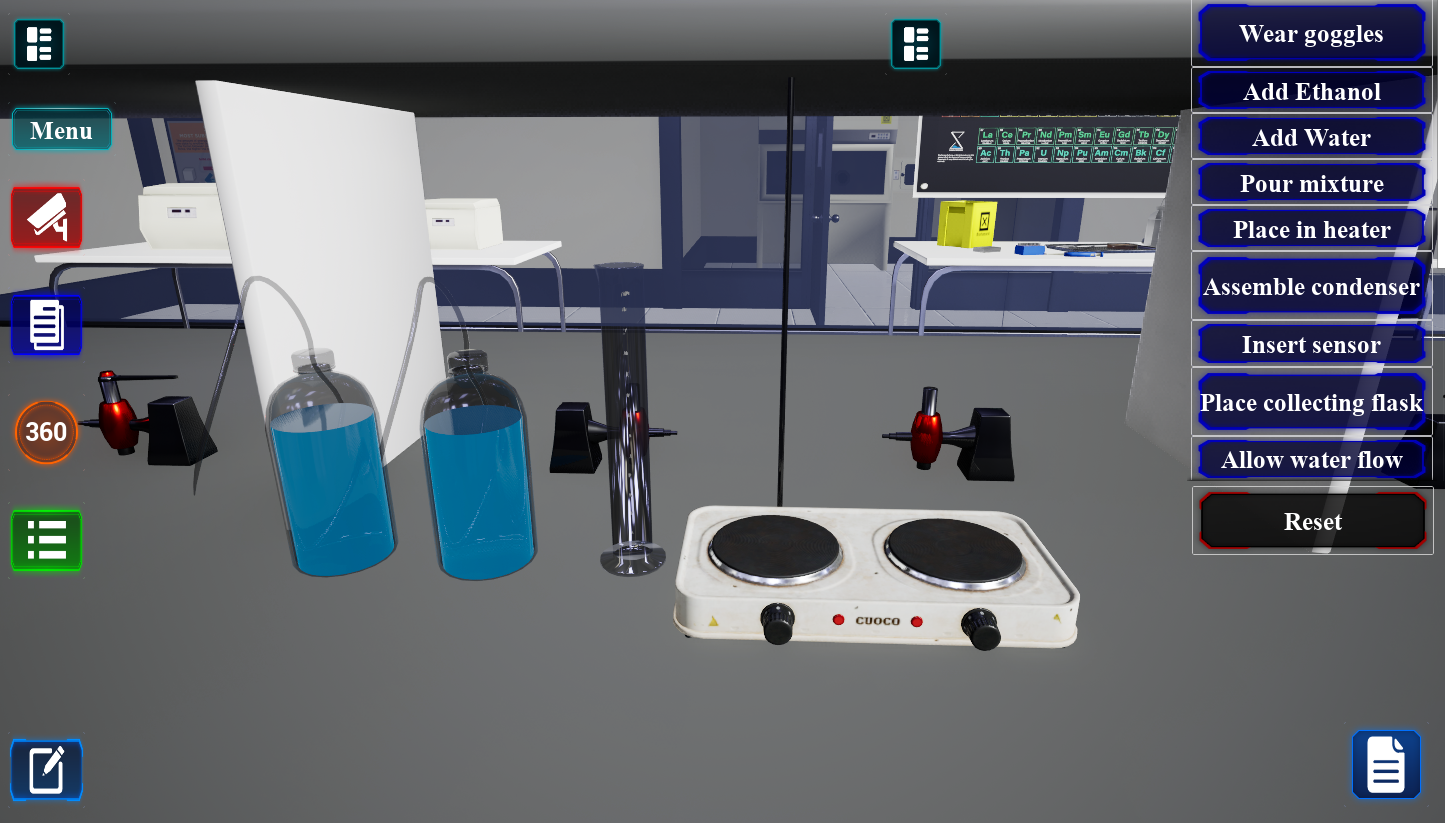
Determining Melting Temperature
Measure melting points to assess purity and identify organic compounds.
30-45 minutesRecrystallization
Purify organic solids by dissolving in hot solvent and allowing slow crystallization.
30-45 minutesDetermination of a Boiling Point
Measure boiling points to characterize liquids and assess purity.
30-45 minutesIdentifying an Unknown Analgesic by Three Methods
Use melting point, TLC, and chemical tests to identify over-the-counter pain relievers.
30-45 minutesSeparation of Organic Compounds by Acid-Base Extraction Techniques
Separate mixtures using differences in acid-base properties and solubility.
30-45 minutesUnderstanding Polarimetry
Learn to measure optical rotation and determine specific rotation of chiral compounds.
30-45 minutesIdentification of Organic Unknowns Using Polarimetry
Use optical rotation measurements to identify unknown chiral organic compounds.
30-45 minutesInvestigating Gas Chromatography
Learn principles and applications of GC for separating and analyzing volatile mixtures.
30-45 minutesFractional Distillation of Esters
Separate ester mixtures based on boiling point differences using fractional distillation.
30-45 minutesUnderstanding Intermolecular Forces Using a Gas Chromatograph: Enthalpy of Vaporization
Use GC retention times to study intermolecular forces and calculate ΔHvap.
30-45 minutesInvestigating Thermodynamic Relationships of Substituted Hydrocarbons
Study how substituents affect boiling points and other physical properties.
30-45 minutesExtraction of Spinach Pigments and Analysis by Electronic Absorption Spectroscopy
Separate plant pigments and analyze their UV-Vis spectra to identify chlorophylls and carotenoids.
30-45 minutesSN1: Synthesis of t-butyl chloride
Prepare tert-butyl chloride via SN1 mechanism and study carbocation stability.
30-45 minutesSN2: Synthesis of 1-bromobutane
Prepare 1-bromobutane via SN2 mechanism and study nucleophilic substitution kinetics.
30-45 minutesObserving the Reaction Kinetics of Sucrose with Polarimetry
Monitor sucrose hydrolysis by measuring changes in optical rotation over time.
30-45 minutesThe Synthesis and Analysis of Aspirin
Prepare acetylsalicylic acid and assess purity through melting point and IR spectroscopy.
30-45 minutesIsolation of R-(+)-Limonene from Oranges using Steam Distillation
Extract and isolate the natural terpene limonene from citrus peels.
30-45 minutesSynthesizing Ethyl Acetate by Fisher Esterification
Prepare ethyl acetate via acid-catalyzed reaction of acetic acid with ethanol.
30-45 minutesSynthesis of Dibenzalacetone by Aldol Condensation
Prepare a conjugated enone through base-catalyzed aldol condensation reaction.
30-45 minutesThe Diels-Alder Reaction of Anthracene with Maleic Anhydride
Perform a cycloaddition reaction to form a bridged polycyclic compound.
30-45 minutesFriedel-Crafts Acylation of Ferrocene
Introduce an acyl group to ferrocene using aluminum chloride catalysis.
30-45 minutesGrignard Formation of Crystal Violet
Synthesize the triphenylmethane dye crystal violet using Grignard reagents.
30-45 minutesSynthesis of Fluorescein
Prepare the fluorescent dye fluorescein through condensation of resorcinol and phthalic anhydride.
30-45 minutesSynthesis of Methyl Orange and Its Application to Textiles
Prepare the azo dye methyl orange and test its dyeing properties on fabric.
30-45 minutesAnalysis of Natural Products
Extract and characterize bioactive compounds from natural sources.
30-45 minutesUsing a Gas Chromatograph: Identifying an Unknown Compound
Use GC retention times and comparison with standards to identify unknown volatile compounds.
30-45 minutes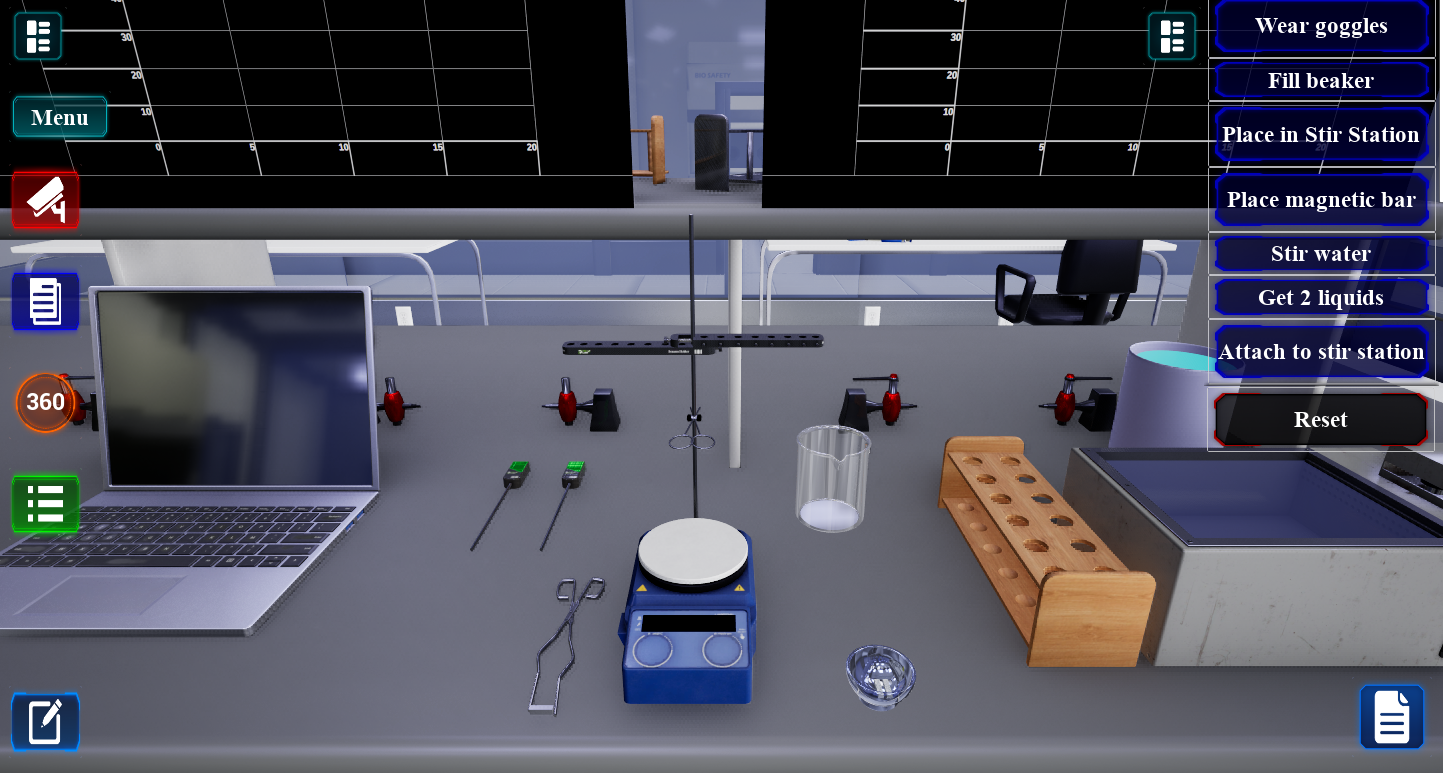
Interactive Learning
Students engage with realistic lab equipment and procedures
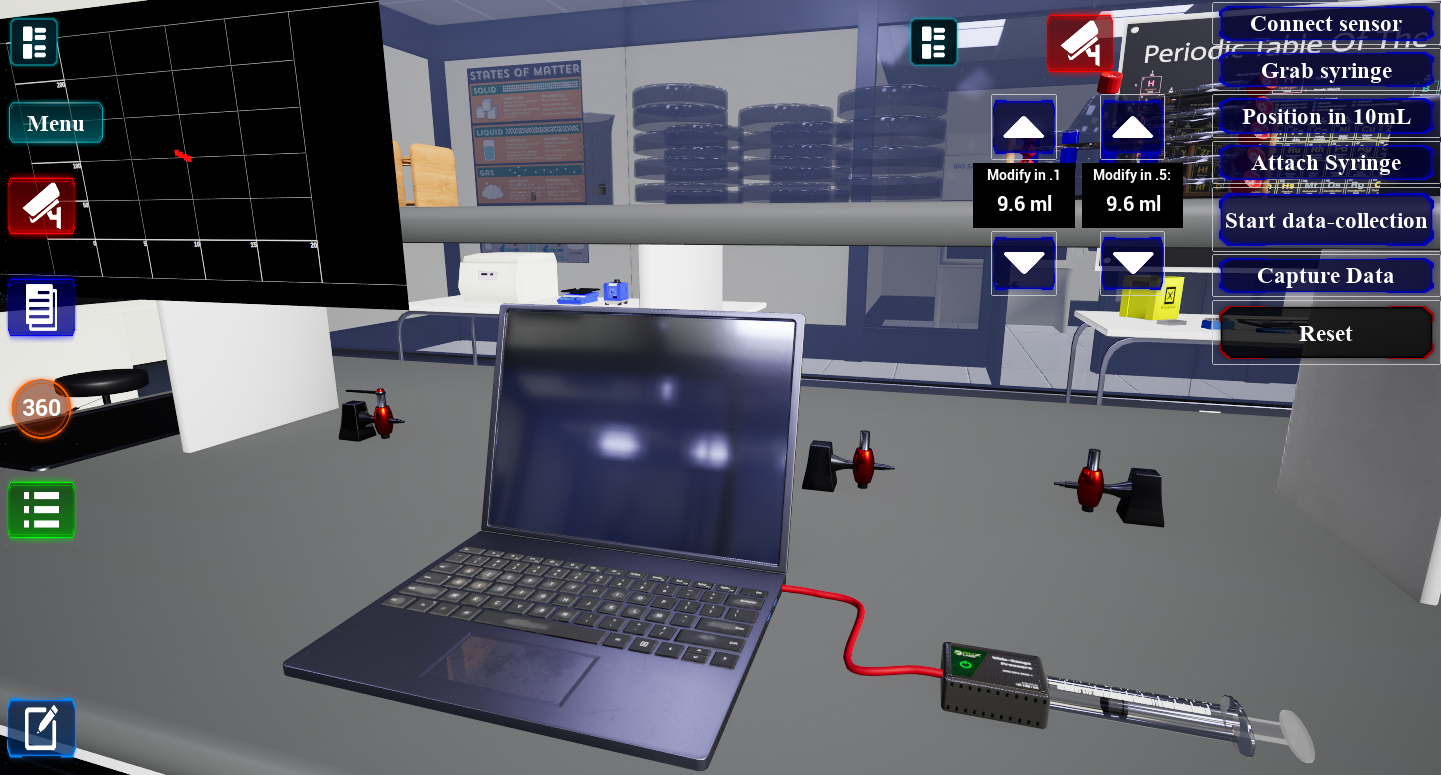
Real-time Data
Collect and analyze data just like in a physical laboratory
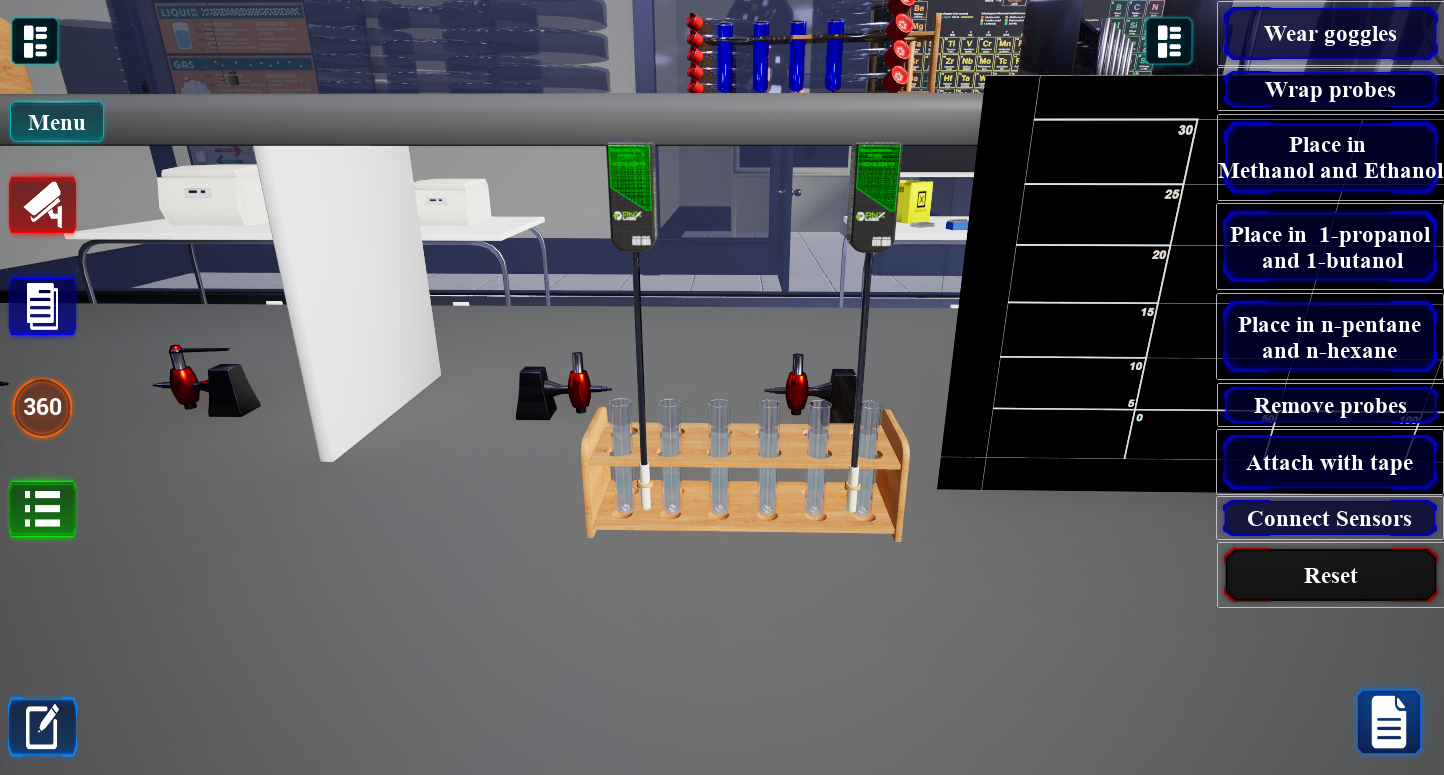
Unlimited Flexibility
Change variables to replicate scenarios that are impossible in a physical lab