

Chemistry 101 Virtual Laboratories
Experience Science Like Never Before
Our virtual laboratories provide students with immersive, hands-on science experiences without the limitations of physical lab spaces. Access cutting-edge experiments and simulations 24/7 from any device.
Each virtual lab is designed by experienced educators and scientists to align with curriculum standards while providing interactive learning opportunities that engage students in authentic scientific inquiry.
Available Virtual Labs
Explore our comprehensive collection of STEM laboratory simulations
Chemistry Laboratories
Endothermic and Exothermic Reactions
Measure temperature changes during chemical processes that absorb or release heat.
30-45 minutesFreezing and Melting of Water
Observe phase changes and measure the freezing/melting point of water.
30-45 minutesAnother Look at Freezing Temperature
Investigate how impurities affect the freezing point of substances.
30-45 minutesHeat of Fusion for Ice
Calculate the energy required to melt ice without changing its temperature.
30-45 minutesFind the Relationship: An Exercise in Graphing Analysis
Develop skills in interpreting experimental data through graphical methods.
30-45 minutesBoyle's Law: Pressure-Volume Relationship in Gases
Verify the inverse relationship between gas pressure and volume at constant temperature.
30-45 minutesPressure-Temperature Relationship in Gases
Investigate Gay-Lussac's Law showing direct proportionality between pressure and temperature.
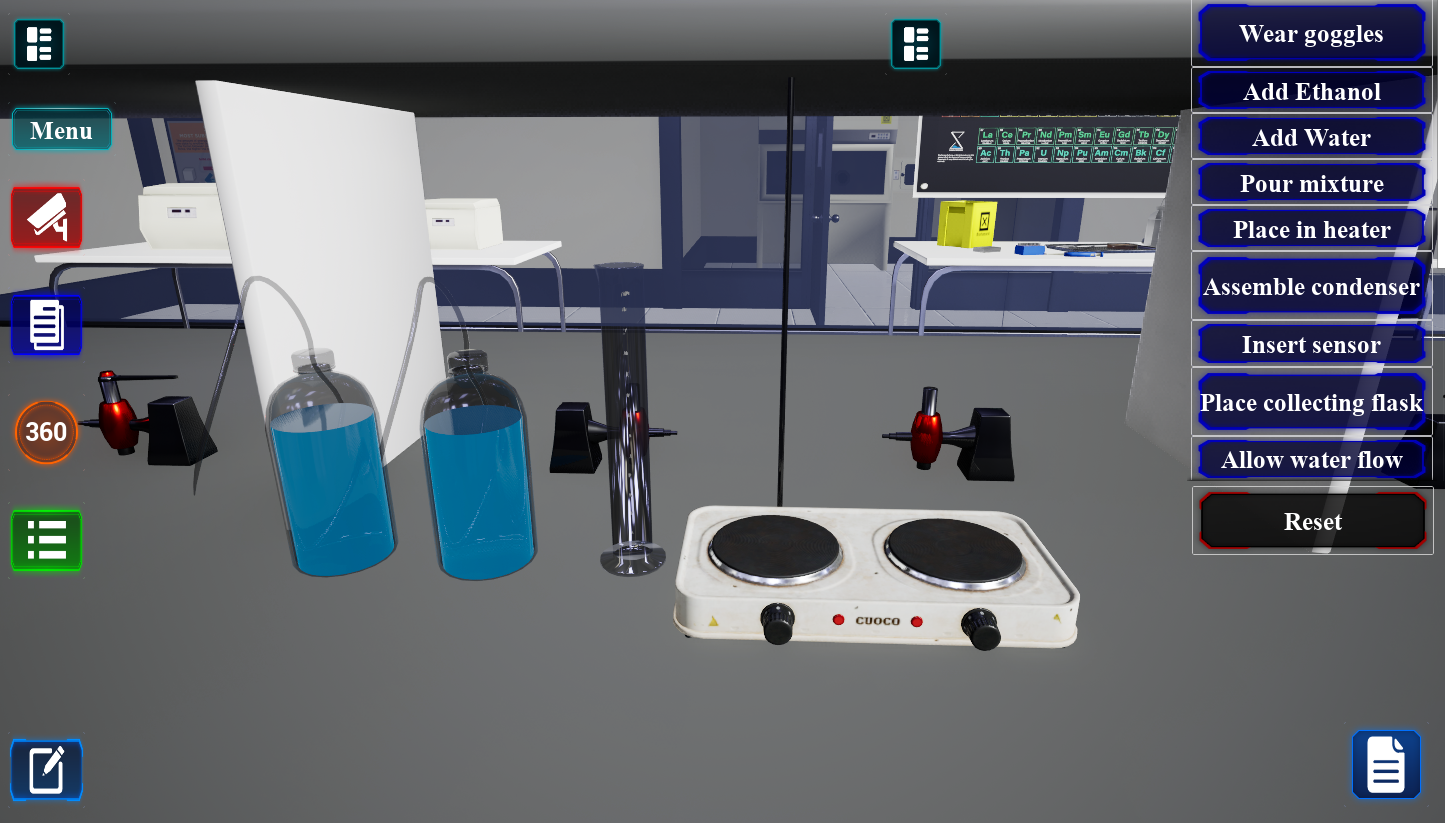
30-45 minutesFractional Distillation
Separate liquid mixtures based on differences in boiling points.
30-45 minutesEvaporation and Intermolecular Attractions
Study how molecular forces affect evaporation rates of different liquids.
30-45 minutesVapor Pressure of Liquids
Measure vapor pressure at different temperatures and understand its relationship with boiling point.
30-45 minutesDetermining the Concentration of a Solution: Beer's Law
Use spectrophotometry to determine unknown concentrations of colored solutions.
30-45 minutesEffect of Temperature on Solubility of a Salt
Investigate how temperature changes affect the maximum amount of solute that dissolves.
30-45 minutesProperties of Solutions: Electrolytes and Non-Electrolytes
Test substances to determine their ability to conduct electricity in solution.
30-45 minutesConductivity of Solutions: The Effect of Concentration
Measure how solution concentration affects electrical conductivity.
30-45 minutesUsing Freezing Point Depression to Find Molecular Weight
Calculate molar mass by measuring how solutes lower freezing points.
30-45 minutesEnergy Content of Foods
Measure the caloric content of different food samples using calorimetry.
30-45 minutesEnergy Content of Fuels
Compare the heat released by combustion of different fuel samples.
30-45 minutesAdditivity of Heats of Reaction: Hess's Law
Verify that enthalpy change is independent of reaction pathway.
30-45 minutesHeat of Combustion: Magnesium
Determine the enthalpy of combustion for magnesium metal.
30-45 minutesChemical Equilibrium: Finding a Constant, Kc
Determine equilibrium constants for reversible reactions.
30-45 minutesHousehold Acids and Bases
Test common household substances to determine their pH and acid-base properties.
30-45 minutesAcid Rain
Simulate and measure the effects of acid rain on different materials.
30-45 minutesTitration Curves of Strong and Weak Acids and Bases
Generate and analyze pH curves to distinguish between strong and weak acid-base systems.
30-45 minutesAcid-Base Titration
Determine unknown concentrations using standardized titrations.
30-45 minutesTitration of a Diprotic Acid: Identifying an Unknown
Analyze titration curves to identify acids with two dissociable protons.
30-45 minutesUsing Conductivity to Find an Equivalence Point
Determine titration endpoints through changes in solution conductivity.
30-45 minutesAcid Dissociation Constant, Ka
Calculate the equilibrium constant for weak acid dissociation.
30-45 minutesEstablishing a Table of Reduction Potentials: Micro-Voltaic Cells
Construct electrochemical cells to measure and compare standard reduction potentials.
30-45 minutesLead Storage Batteries
Study the chemistry and performance characteristics of lead-acid batteries.
30-45 minutesRate Law Determination of the Crystal Violet Reaction
Determine reaction order and rate constant through colorimetric analysis.
30-45 minutesTime-Release Vitamin C Tablets
Analyze the dissolution kinetics of controlled-release vitamin formulations.
30-45 minutesThe Buffer in Lemonade
Investigate the buffering capacity of citric acid systems in beverages.
30-45 minutesDetermining the Free Chlorine Content of Swimming Pool Water
Use colorimetric titration to measure chlorine concentration in water samples.
30-45 minutesDetermining the Quantity of Iron in a Vitamin Tablet
Use spectrophotometry to quantify iron content in commercial supplements.
30-45 minutesDetermining the Phosphoric Acid Content in Soft Drinks
Titrate cola beverages to measure their phosphoric acid concentration.
30-45 minutesMicroscale Acid-Base Titration
Perform precise titrations using small reagent volumes and specialized equipment.
30-45 minutes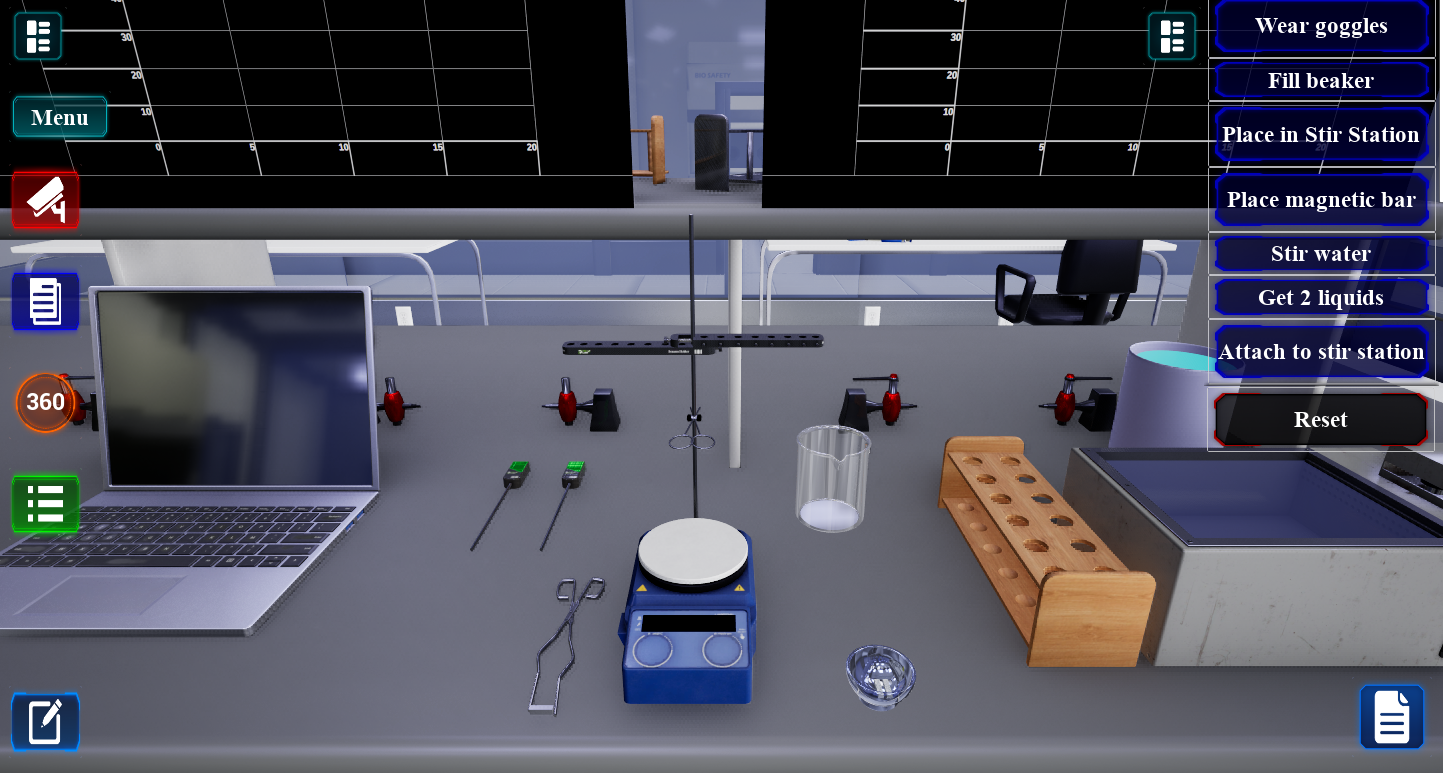
Interactive Learning
Students engage with realistic lab equipment and procedures
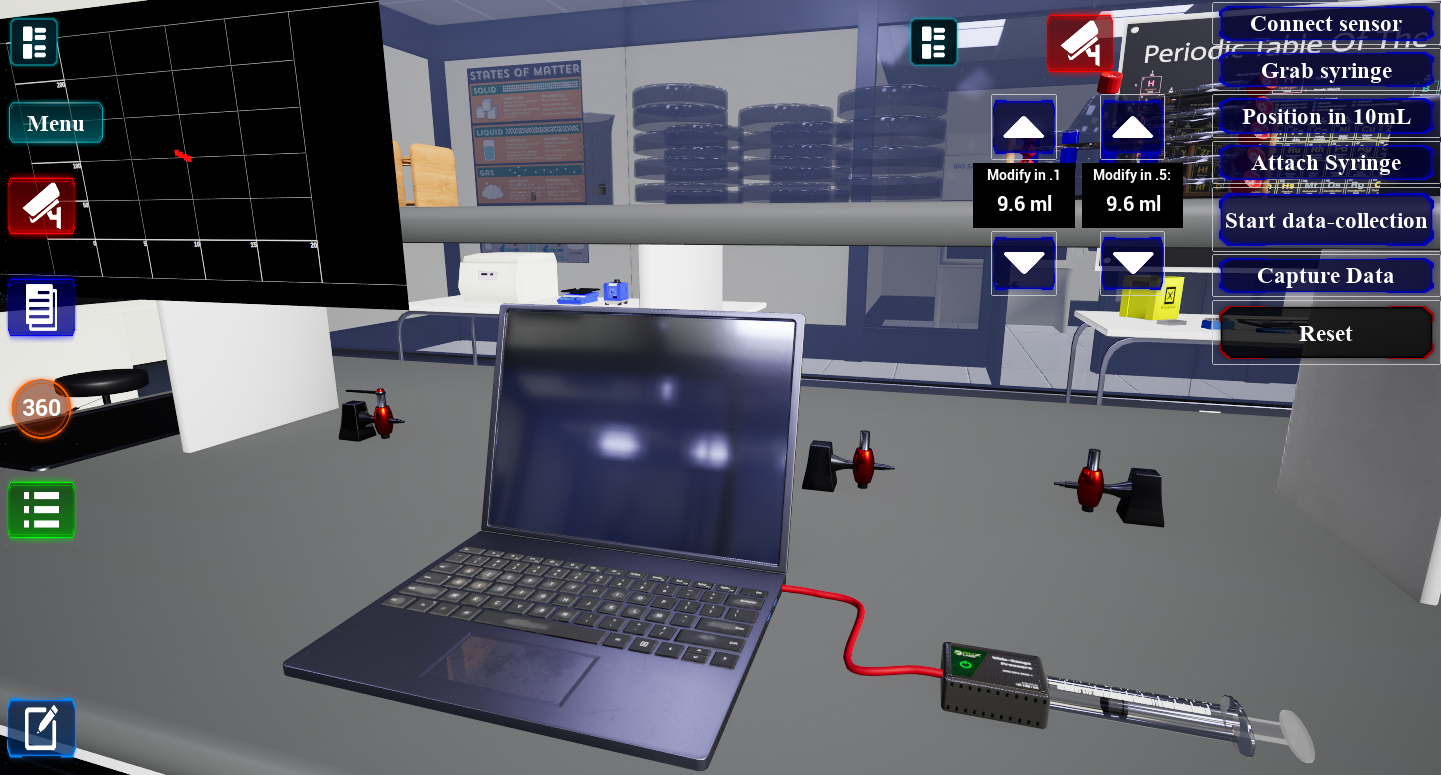
Real-time Data
Collect and analyze data just like in a physical laboratory
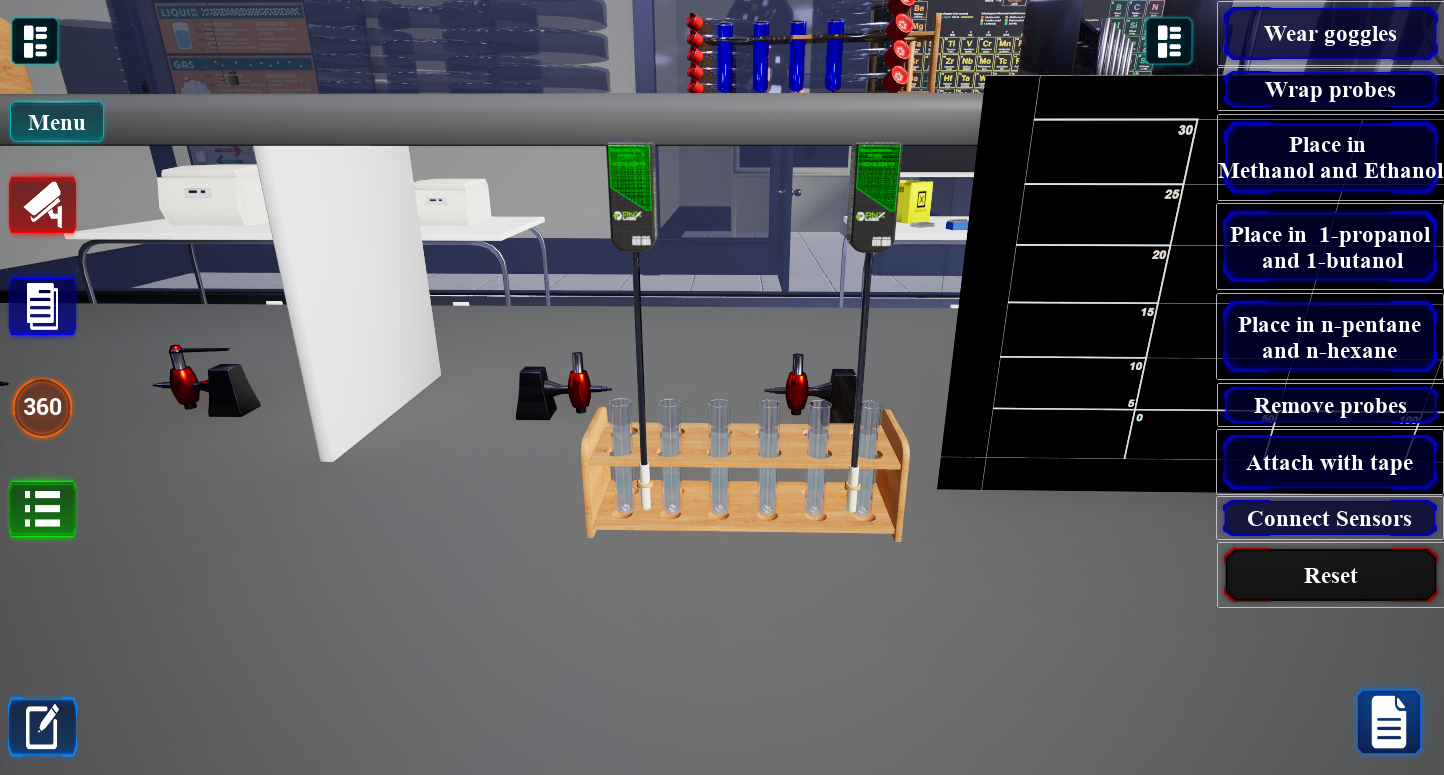
Unlimited Flexibility
Change variables to replicate scenarios that are impossible in a physical lab