
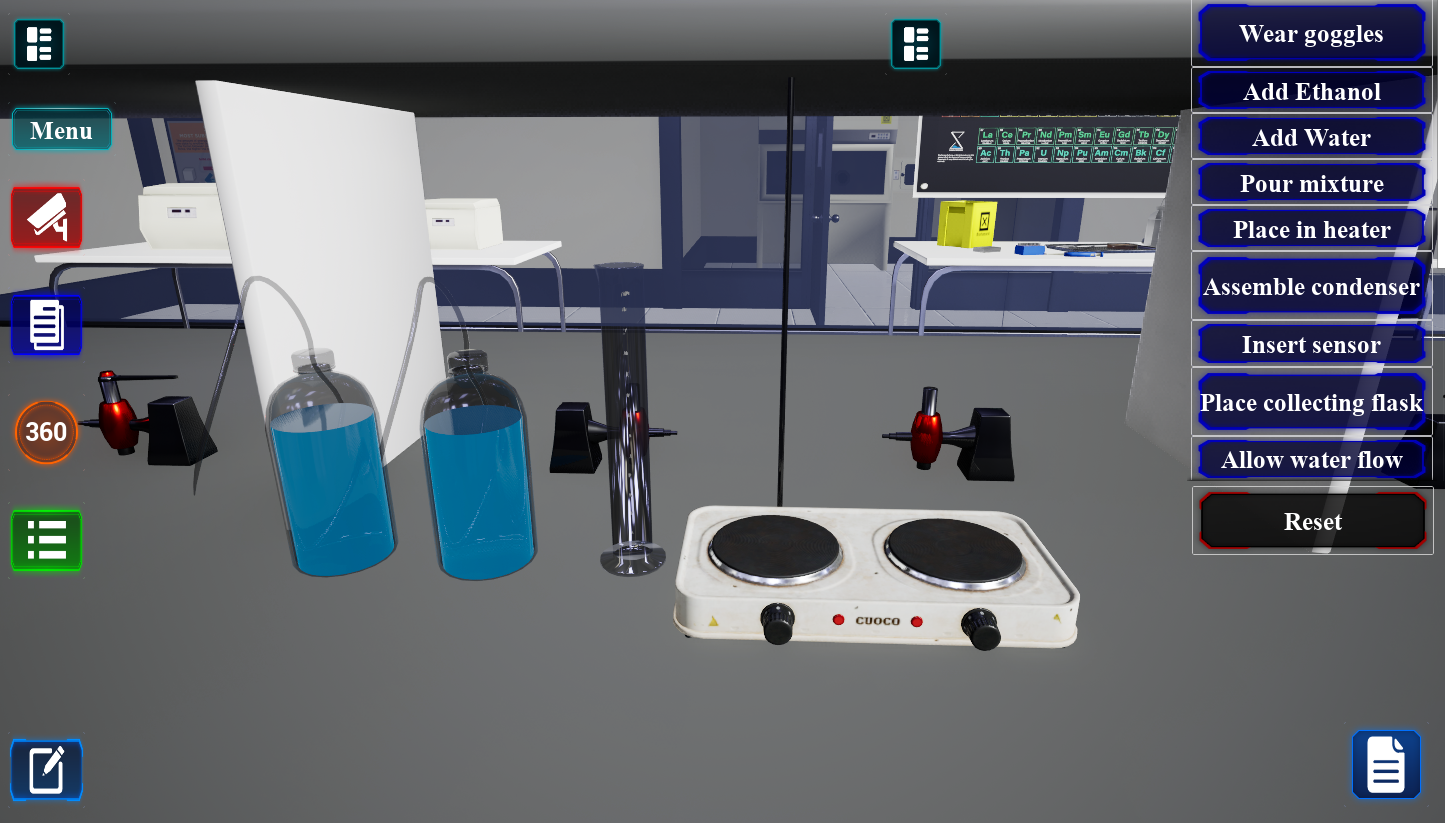

Chemistry 102 Virtual Laboratories
Experience Science Like Never Before
Our virtual laboratories provide students with immersive, hands-on science experiences without the limitations of physical lab spaces. Access cutting-edge experiments and simulations 24/7 from any device.
Each virtual lab is designed by experienced educators and scientists to align with curriculum standards while providing interactive learning opportunities that engage students in authentic scientific inquiry.
Available Virtual Labs
Explore our comprehensive collection of STEM laboratory simulations
Chemistry Laboratories
The Determination of a Chemical Formula
Use experimental data to determine the empirical formula of a compound.
30-45 minutesThe Determination of the Percent Water in a Compound
Measure water content in hydrated salts using heating and weighing techniques.
30-45 minutesThe Molar Mass of a Volatile Liquid
Use the Dumas method to determine molecular weight through vapor density measurements.
30-45 minutesUsing Freezing-Point Depression to Find Molecular Weight
Determine molar mass by measuring how solutes lower freezing points.
30-45 minutesThe Molar Volume of a Gas
Determine the volume occupied by one mole of gas at standard temperature and pressure.
30-45 minutesStandardizing a Solution of Sodium Hydroxide
Prepare and standardize NaOH solution using primary standard potassium hydrogen phthalate.
30-45 minutesAcid-Base Titration
Determine unknown acid or base concentrations through neutralization reactions.
30-45 minutesAn Oxidation-Reduction Titration: The Reaction of Fe2+ and Ce4+
Use redox titration to determine iron concentration using cerium(IV) as titrant.
30-45 minutesDetermining the Mole Ratios in a Chemical Reaction
Use stoichiometric methods to find reactant ratios in chemical equations.
30-45 minutesThe Determination of an Equilibrium Constant
Measure concentrations at equilibrium to calculate Kc for chemical systems.
30-45 minutesInvestigating Indicators
Study pH indicators and their color changes at different hydrogen ion concentrations.
30-45 minutesThe Decomposition of Hydrogen Peroxide
Study catalyst effects and measure reaction rates of H₂O₂ breakdown.
30-45 minutesDetermining the Enthalpy of a Chemical Reaction
Use calorimetry to measure heat changes in chemical reactions.
30-45 minutesSeparation and Qualitative Analysis of Cations
Use selective precipitation and chemical tests to identify metal ions.
30-45 minutesSeparation and Qualitative Analysis of Anions
Identify negative ions through characteristic chemical reactions.
30-45 minutesThe Synthesis of Alum
Prepare potassium aluminum sulfate dodecahydrate from aluminum metal.
30-45 minutesThe Analysis of Alum
Verify alum composition through gravimetric and other analytical techniques.
30-45 minutesConductimetric Titration and Gravimetric Determination of a Precipitate
Combine conductivity measurements with precipitate weighing for quantitative analysis.
30-45 minutesDetermining the Concentration of a Solution: Beer's Law
Use spectrophotometry to determine unknown concentrations from absorbance measurements.
30-45 minutesLiquid Chromatography
Separate mixture components based on differential partitioning between mobile and stationary phases.
30-45 minutesBuffers
Prepare and test buffer solutions and measure their resistance to pH changes.
30-45 minutesElectrochemistry: Voltaic Cells
Construct galvanic cells and measure their voltage to study redox potentials.
30-45 minutesElectroplating
Use electrolysis to deposit metal coatings on conductive surfaces.
30-45 minutesThe Synthesis and Analysis of Aspirin
Prepare acetylsalicylic acid and assess purity through melting point and titration.
30-45 minutesDetermining the Ksp of Calcium Hydroxide
Calculate the solubility product constant for calcium hydroxide through titration.
30-45 minutesDetermining Ka by the Half-Titration of a Weak Acid
Calculate acid dissociation constant at the half-equivalence point of a titration.
30-45 minutesThe Rate and Order of a Chemical Reaction
Determine reaction order and rate constant through concentration-time studies.
30-45 minutesThe Enthalpy of Neutralization of Phosphoric Acid
Measure heat released when phosphoric acid is neutralized by a strong base.
30-45 minutesα, β, and γ
Study properties of different types of nuclear radiation and their interactions with matter.
30-45 minutesRadiation Shielding
Test effectiveness of different materials in blocking various types of radiation.
30-45 minutesThe Base Hydrolysis of Ethyl Acetate
Study saponification kinetics of ethyl acetate with sodium hydroxide.
30-45 minutesExploring the Properties of Gases
Investigate gas laws and relationships between pressure, volume, and temperature.
30-45 minutesDetermining Avogadro's Number
Calculate Avogadro's number through electrolysis or monolayer techniques.
30-45 minutesPotentiometric Titration of Hydrogen Peroxide
Use electrode potential measurements to determine hydrogen peroxide concentration.
30-45 minutesDetermining the Half-Life of an Isotope
Measure radioactive decay to calculate the half-life of a radioisotope.
30-45 minutesVapor Pressure and Heat of Vaporization
Measure vapor pressure at different temperatures to calculate enthalpy of vaporization.
30-45 minutesRate Determination and Activation Energy
Study temperature effects on reaction rates to calculate activation energy.
30-45 minutes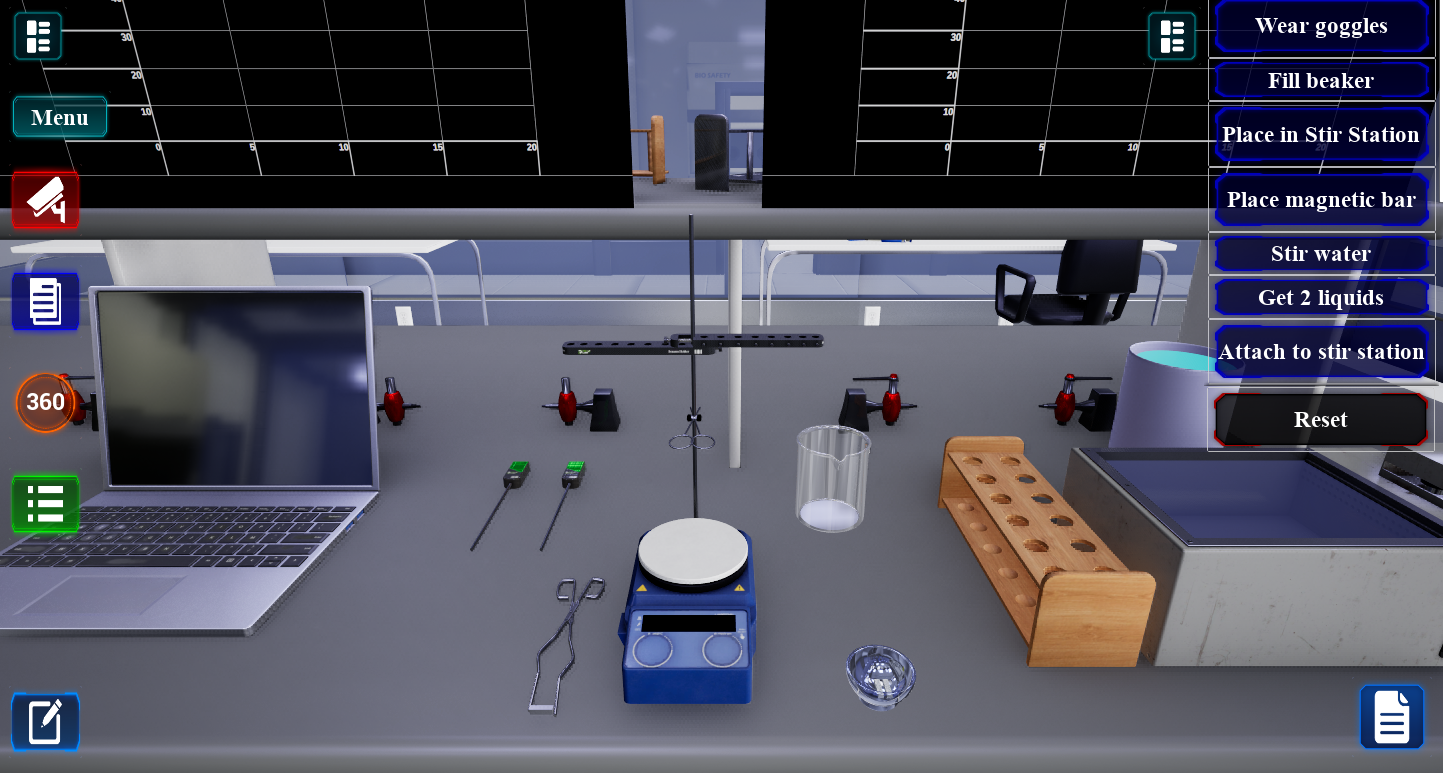
Interactive Learning
Students engage with realistic lab equipment and procedures
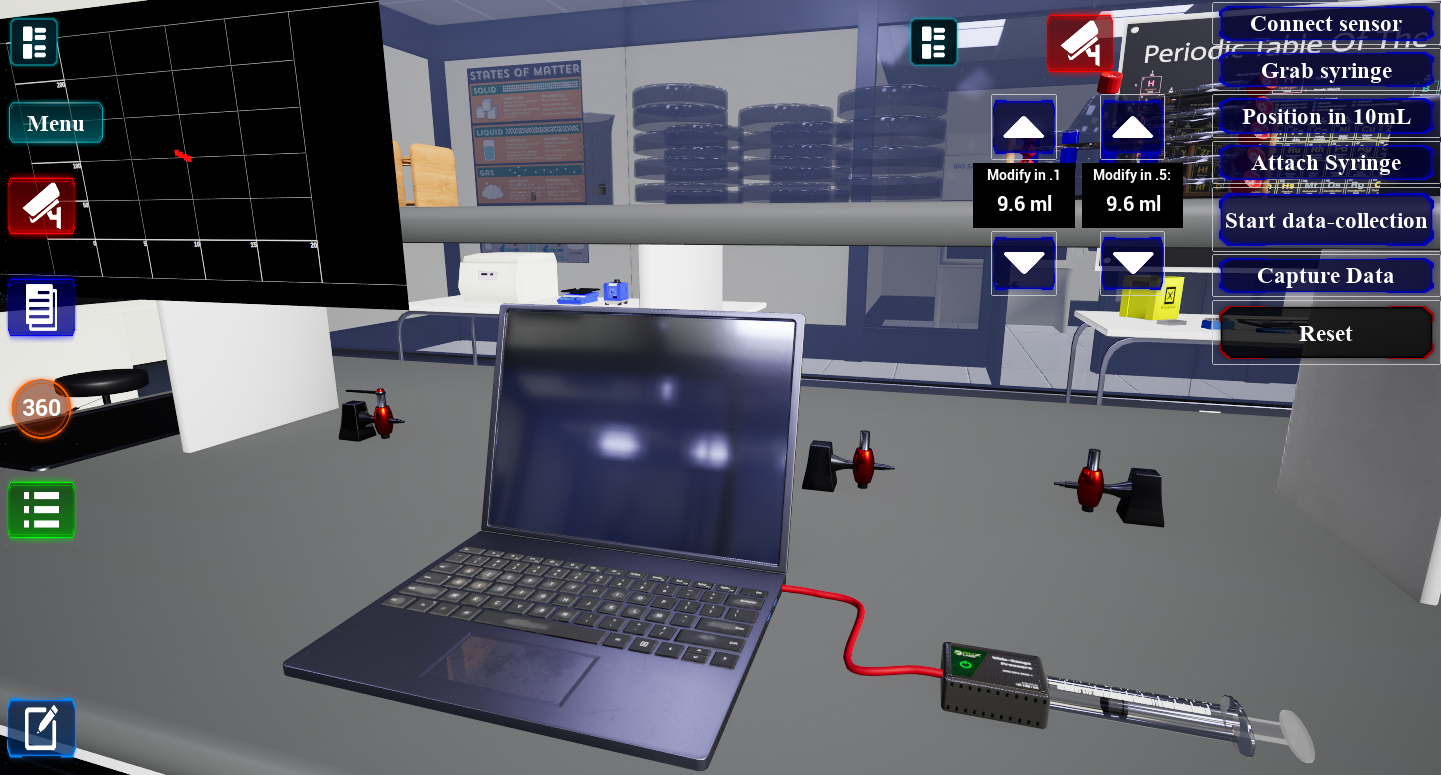
Real-time Data
Collect and analyze data just like in a physical laboratory
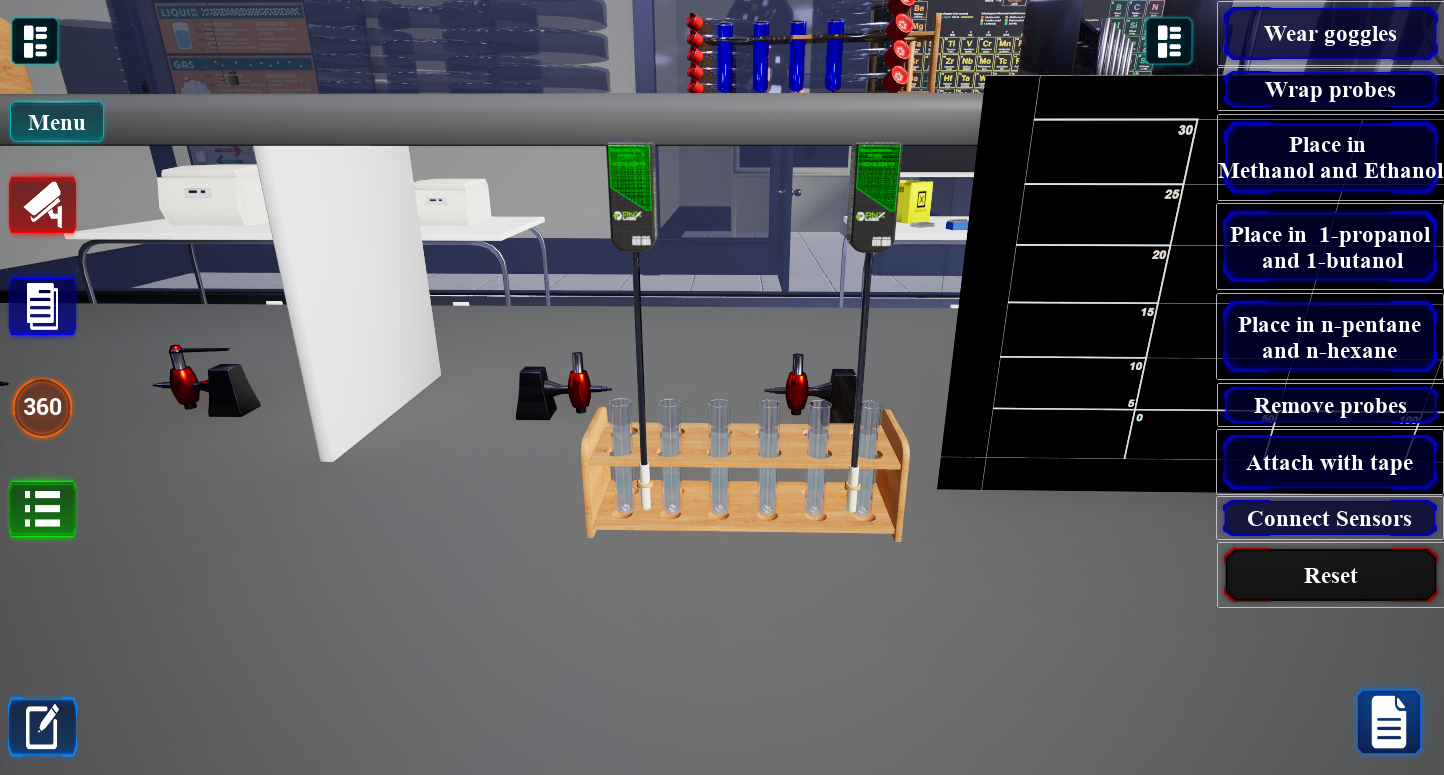
Unlimited Flexibility
Change variables to replicate scenarios that are impossible in a physical lab